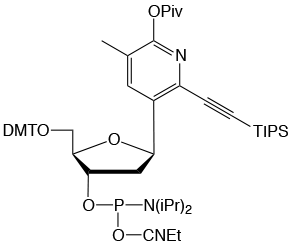
dW is a C-nucleoside that acts as a strong adenine base paring analog.
In addition to the typical two hydrogen bonds found between T and A, dW
can also interact with A via van der Waals forces. The result is a dW–A
interaction that approaches the strength of a C–G base pair while also
exhibiting enhanced base-pairing fidelity. dW can be used in place of T
as a single substitution or a complete replacement for oligonucleotide
hybridization applications. Please see Glen Report article 31.21 and Glen Report article 31.22 for more details.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200